Propublica is a nonprofit newsroom that investigates power abuse. Sign up and receive the biggest story as soon as it’s published.
The Food and Drug Administration has discovered a problem at a factory in India. It will produce generic drugs in American patients, including one drug manufactured there and associated with at least eight deaths, federal records show.
After a Propublica investigation in December, the agency inspected the factory. It turns out that the factory operated by Glenmark drugs is responsible for a large share of tablet recalls that do not melt properly and can cause harm to people. In a series of recalls, the FDA determined last year that more than 50 million potassium chloride expanded release capsules could kill US patients.
Still, Propublica discovered that the FDA had not dispatched inspectors to its factory in Madhya Pradesh, India.
When FDA inspectors went to the Glenmark factory last month – five years after the agency’s pre-examination, they discovered cleaning and testing issues that they said could affect the drugs shipped to American consumers.
In a report detailing the findings, inspectors wrote that Glenmark was unable to resolve the reason why some of the drugs did not dissolve properly, raising concerns about the factory’s manufacturing process.
What we see
During Donald Trump’s second presidency, Propovica will focus on areas that need scrutiny. Below are some of the issues reporters watch, and how to safely communicate with them.
We are doing something new. Helpful?
“Equipment and cooking utensils will not be cleaned at appropriate intervals to prevent contamination that changes the safety, identity, strength, quality or purity of the medicine,” the inspector wrote.
The FDA compiled a large strip of testing reports, making it impossible for inspectors to determine whether the tablets were uncovering why they weren’t dissolved correctly, or whether Glenmark drugs sitting in American drug cabinets could be at risk of contamination.
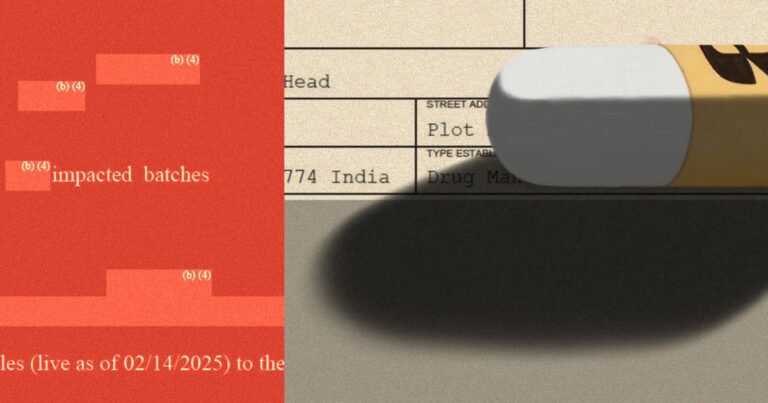
Propublica obtained the report through the Freedom of Information Act. To justify censorship of the document, FDA lawyers cited trade secrets.
Sold by the day before the test ended, Health and Human Services Director Robert F. Kennedy Jr. vowed to bring “radical transparency” to his agency overseeing the FDA. Propublica asked the HHS media team if Kennedy believes that heavily edited inspection records are in line with his promise of transparency, and if he believes that the name of the drug that the inspector raised safety concerns is a trade secret. The media team did not respond.
An FDA spokesperson wouldn’t say why the agency had been waiting so long to inspect the plant, or, if any, what federal regulators would call for Glenmark to resolve the issue. “The FDA generally cannot discuss potential or continuing compliance issues except for the companies involved,” she writes.
A review by the Glenmark plant by the FDA stated that “it is a test that can be caused if there is reason to believe that the facility has a quality issue, and if there is reason to follow up with complaints or other reasons.
Drugs that cannot be properly dissolved can cause dangerous shaking during administration. Since Glenmark’s potassium chloride was recalled in May, the company has been reported to federal regulators of eight deaths of people who took the recalled capsules, FDA records show. Companies are required to submit a report of adverse events received from the patient or their physician. This allows agencies to monitor drug safety. However, the FDA shares most of the details. As a result, Propublica was unable to independently verify what happened in each of these cases. Generally, the FDA says these reports reflect the opinions of people who report harm and do not prove that it was caused by the drug.
The family of the 91-year-old Maine woman sued Glenmark in federal court in Newark, New Jersey last year, claiming that potassium chloride was liable for her death in June. In court filings, the company denied liability.
A spokesman for Mumbai-based Glenmark refused to answer detailed questions about the testing, citing the ongoing lawsuit. “Glenmark is still committed to working diligently with the FDA to ensure manufacturing operations and compliance with quality systems,” the spokesman wrote.
Glenmark’s managing director told investors and analysts last month about revenue calls that between 25% and 30% of US revenue came from drugs produced at the Madhya Pradesh plant.
The inspector visited the factory between February 3rd and February 14th. Similar to such reports, this point to the observations of inspectors “does not represent the final agency’s decision” regarding compliance with the FDA’s drug manufacturing regulations.
Glenmark did not have a proper cleaning procedure to prevent one drug residue from being rolled up in the next batch of tablets produced on the same machine, inspectors found. Glenmark refused three batches when the test found cross contamination, but inspectors said the same equipment was used to make other drugs shipped to the US.
The FDA has largely compiled the first four pages of its report on visits to factories run by Glenmark drugs. Credit: Get by Propublica
Propublica asked the FDA if the agency was testing any of these drugs for contamination. The spokesman did not refer the reporter to the FDA website, which displays past test results but has not been included in Glenmark products since the recall, instead referred them instead.
Vice President of Glenmark, who is in charge of quality, said that the major production equipment has not been decontaminated before the company uses it to make some medicines. It is unclear what these drugs are, as the FDA censored some of the reports.
Inspectors noted that Glenmark received two consumer complaints about side effects on one of the drugs. When Glenmark investigated the complaints, the company was unable to assess potential issues that could arise when drugs were manufactured using shared facilities and equipment, the report said. However, the name of the drug and the type of contamination that inspectors were concerned about were not clear due to the compilation of the FDA.
Glenmark also failed to reach the bottom of why some of the drugs made at the factory did not dissolve properly, FDA inspectors found. The company’s investigation into several batches of drugs with disabilities did not identify any specific root causes. The reason for identifying the reason was not properly supported by the evidence or did not explain all the data, the inspector wrote.
Inspectors also raised concerns that several drugs made in the factory and the key ingredients that enter them are “released on a daily basis by testing using analytical testing methods that have not been properly verified or verified.” Inspectors listed those currently in the US market, but the FDA edited the names of the drugs.
When tests from Glenmark analysts discovered drug problems, the company sometimes invalidated these results and “retested with a new sample to get a successful result,” the FDA report states. “The batch was eventually released to the US market.”
Glenmark has been subject to FDA scrutiny for many years. Since 2019, government agency inspectors have discovered major flaws in three of the four factories of the company that manufactured drugs for American patients. One plant issue was so bad that in 2022 government agencies banned drugs that were prohibited from entering the US
The FDA has not inspected the drug factory after recalling the same defect seven times. 1 Potentially fatal
The string of recalls originating from products made at the Madhya Pradesh factory in central India began in October 2023. For the next 12 months, a single plant may not properly dissolve more than 30% of all FDA recalls and harm patients.
The federal government often doesn’t make it easy for consumers to know where their medicine is produced. To identify this pattern, Propublica had to match details of the two FDA databases with the US National Library of Medicine drug label records.
While the majority of factories that make drugs for American patients are abroad, Congress’s investigative department has repeatedly discovered that there are too few inspectors to properly oversee the FDA.