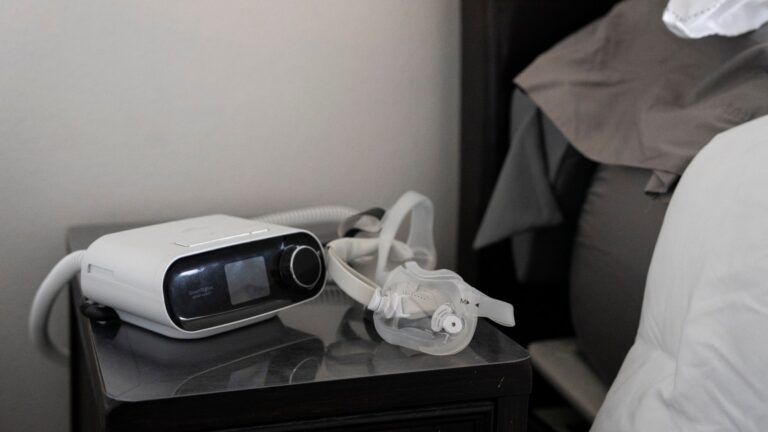
The Food and Drug Administration rarely uses its authority to remove dangerous medical devices from the market and is so understaffed that it sometimes cannot verify whether companies are taking important steps to protect patients during health emergencies, according to a new report from the General Accounting Office.
The congressional watchdog investigation was requested by Senate leaders nearly two years ago in the wake of an investigation into a 2021 respiratory recall that threatened the health of millions of Americans.
ProPublica and the Pittsburgh Post-Gazette revealed that the FDA has received hundreds of complaints about the machine over the years, but has never ordered a recall. Philips Respironics ultimately recalled the device, which is fitted with industrial foam that can break down and release toxic substances into the lungs of patients, including the elderly, veterans, and young children.
According to news outlets, Philips filed thousands more complaints before initiating the recall, but did not notify the FDA. Customers who relied on continuous positive airway pressure machines (CPAP), machines, and other devices reported respiratory illnesses, kidney and liver conditions, and cancer.
In 2023, U.S. Sens. Dick Durbin, D-Ill., and Richard Blumenthal, D-Conn., asked the GAO to investigate GAO’s practices during one of the most tumultuous medical device recalls in recent history.
Durbin said in a statement that GAO’s report is “long overdue” and details “the harm caused by deep staff reductions” and “how weakened enforcement authority makes it difficult for FDA to carry out important oversight activities.”
The investigation, which began last year, found that the FDA did not use its authority to force manufacturers to pull defective devices from the market. Although the agency has been authorized to take such actions for decades under federal law, it has done so only four times, the last time in 1992.
According to the GAO, equipment manufacturers have voluntarily initiated recalls in approximately 900 cases in each of the past five years, a staggering number.
Even if companies begin the process, FDA officials, who have weathered deep cuts under the Trump administration, can’t always adequately ensure there are no delays or mistakes in removing defective devices or communicating with consumers, the report said.
According to GAO, the agency currently regulates more than 190,000 medical devices in the United States, an increase of about 15,000 since 2016.
“FDA needs additional resources and staff to adequately protect Americans from unsafe medical devices,” Blumenthal said in a statement. “FDA’s current and future workforce reductions will only further undermine FDA’s ability to protect people from unnecessary harm.”
An investigation by ProPublica and the Post-Gazette found that some patients didn’t know about the 2021 respiratory recall for months or even years and continued using it even though company tests found dangerous compounds were being released from the bubbles in the ventilators.
The agency has received more than 500 reports of deaths related to the device since 2021, according to the FDA’s latest update.
Dr. Rita Redberg, a cardiologist and medical device safety expert, called the FDA’s recall notification system “rudimentary by today’s standards” and said improvements have been desperately needed for years.
“This means that recall notices are still sent by fax because the FDA lacks a modern, comprehensive data infrastructure for recalls,” she said. “I’m really shocked.”
One former FDA official said he was concerned that the agency’s recall response would become less efficient following recent staff cuts. FDA officials told GAO that they are often unable to perform basic tasks such as reviewing status reports submitted by companies while a recall is in progress. These reports detail the number of people notified of a defective device, the number of products repaired, and the estimated time period for the recall to be completed.
Dr. Peter Lurie, a former FDA deputy commissioner who left the agency in 2017, said, “I’m concerned that the situation at FDA is even worse than what is portrayed in the report.”
Following GAO’s findings, the Department of Health and Human Services, which oversees the FDA, said it would evaluate the need for additional personnel and stronger legislative authority to better manage recalls. The FDA previously defended its response to Philips’ recall, saying it acted quickly after learning of the safety concerns.
Phillips, which manufactured the device at two factories outside Pittsburgh, said the original foam caused “no appreciable harm” to patients. Last year, the company reached an agreement with the Justice Department to hire an independent safety monitor and commit to regular inspections of its facilities.
The company also agreed to pay more than $1 billion to settle lawsuits brought by thousands of people who say they were harmed by the device. Under the terms of the settlement, Phillips did not admit fault or liability.
This week, Mr. Durbin and Rep. Jan Schakowsky (D-Ill.) introduced legislation that would require the FDA to address some of GAO’s most pressing concerns.
Among other things, the bill would require the FDA to establish an electronic format for recall alerts to more easily share critical information between companies, the FDA, hospitals, and physicians.
“Millions of Americans rely on medical devices to stay healthy,” Durbin said. “However, if a medical device is recalled, patients have a right to know as soon as possible to understand the risks.”